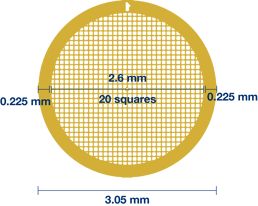
There are many publications that describe the process of cryo-EM sample preparation. These include:
- Passmore and Russo. Specimen preparation for high-resolution cryo-EM. Meth. Enzym. 579: 51-86 (2016).
- Drulyte et al. Approaches to altering particle distributions in cryo-electron microscopy sample preparation. Acta Cryst D74: 560-571 (2018).
- White et al. Single Particle Cryo-Electron Microscopy: From Sample to Structure. J Vis Exp 171 (2021).
In addition there are many online resources providing detailed information about sample preparation including: